Homology Modeling
1 Modeling protein structures: Mind the gap
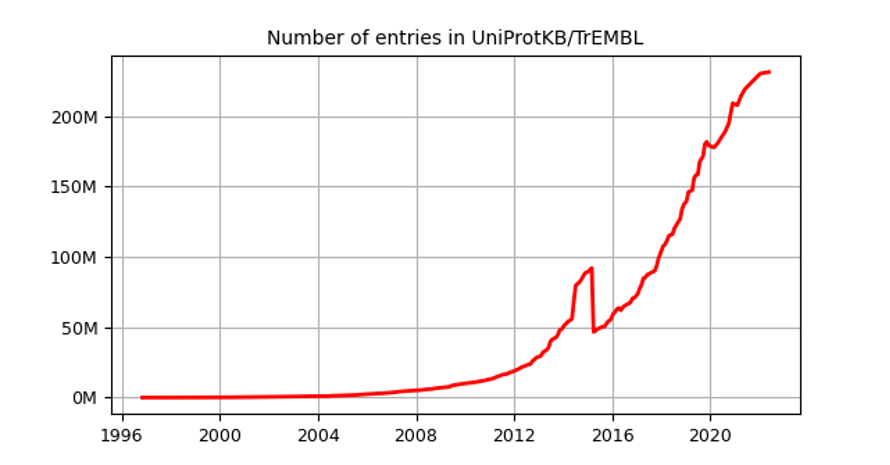
The number of protein sequences in databases growth exponentially during the last decades, particularly after the revolution of high throughput sequencing methods.
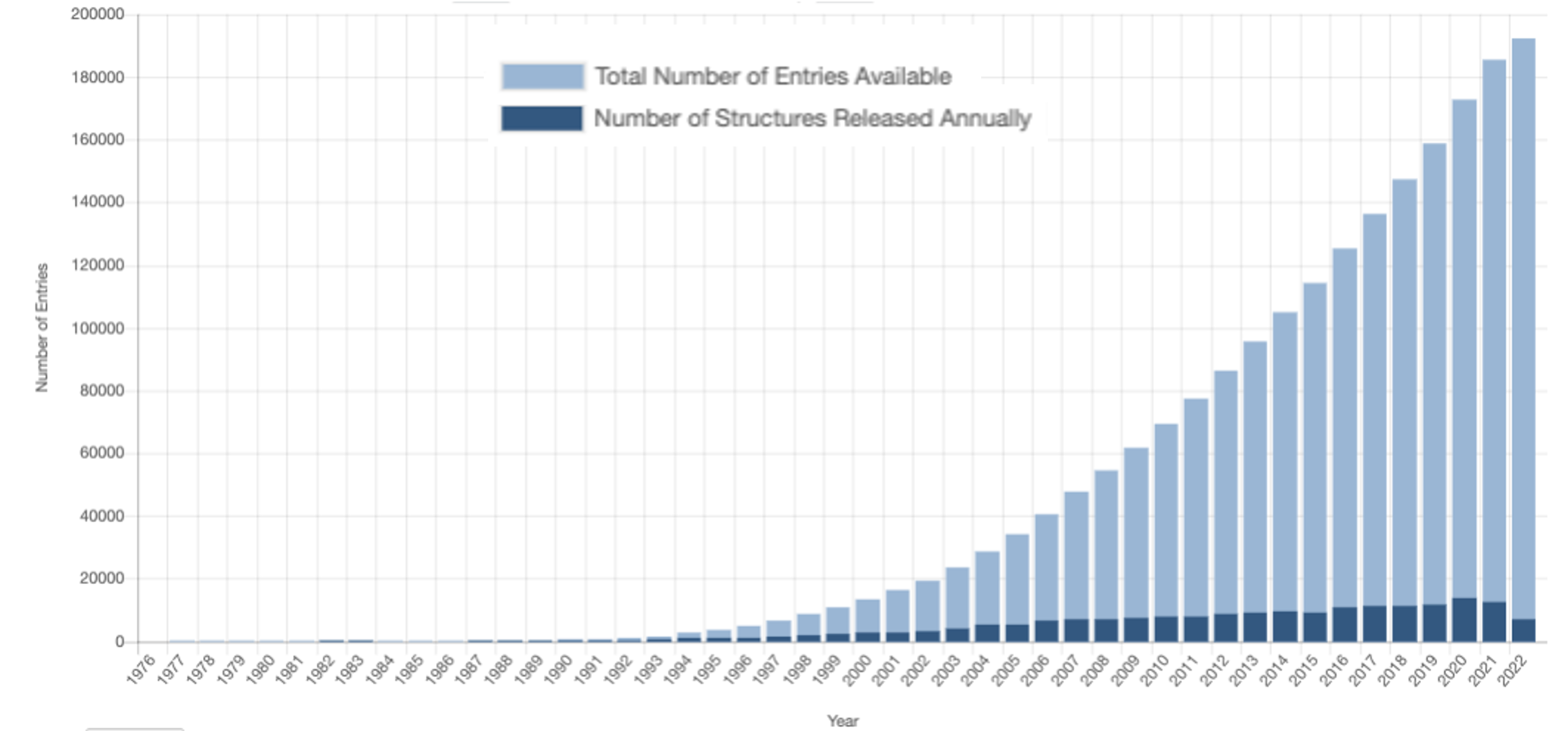
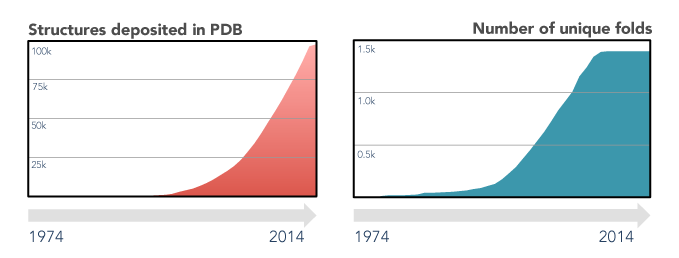
However, experimental determination of 3D protein structures is often difficult, time-consuming, and subjected to limitations, such as experimental error, data interpretation and modeling new data on previously released structures. Thus, despite substantial efforts that started at the beginning of the 21st century to implement high-throughput structural biology methods (see for instance Manjasetty et al. 2008), the availability of protein structures is more than 1,000 times less than the number of sequences (231,354,261 sequences for Uniprot and 192,489 structures in RCSB Protein Databank in 2022). This difference is called the protein structure gap and it is constantly widening (Muhammed and Aki-Yalcin 2019).
Thus, an accurate prediction of the 3D structure of any given protein is needed to make up for the lack of experimental data.
1.1 Are protein structures predictable at all?
Amino acid properties determine the phi and psi angles that eventually shape the higher structural levels. Protein folding might be more complex though, as it should be coupled to protein synthesis.
In 1968, Cyrus Levinthal (1922–1990) published the so-called Levinthal’s paradox, stating that proteins fold in nano/milliseconds, but even for small peptides it will take a huge time to test the astronomical number of possible conformations. Say a 100 aa small protein; it will have 99 peptidic bonds and 198 different phi and psi angles. Assuming only 3 alternative conformations for each bond, it will yield 3198 (= 2.95 x 1095) possible conformations. If we design a highly efficient algorithm that tests 1 conformation per nanosecond:
2.95 x 1085 secs = 9x1067 billions years
Considering that the age of the universe is 13.8 billion years, predicting protein structures does not seem an easy task.
In this context, roughly 50 years ago, a very simple experiment led some light on the protein folding mechanism. Cristian Anfisen was able to completely denature (unfold) the Ribonuclease A, by the addition of reducing agents and urea under heat treatment, and subsequently switch to normal conditions that allow the protein to re-fold fully functional. This experiment indicates that the amino acid sequence dictates the final structure. Notwithstanding some relevant exceptions, this has been largely confirmed.
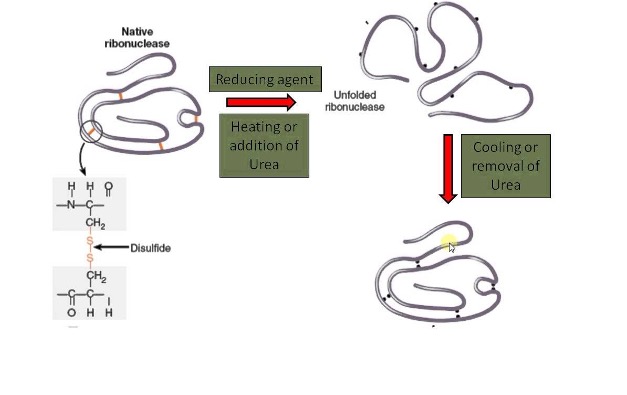
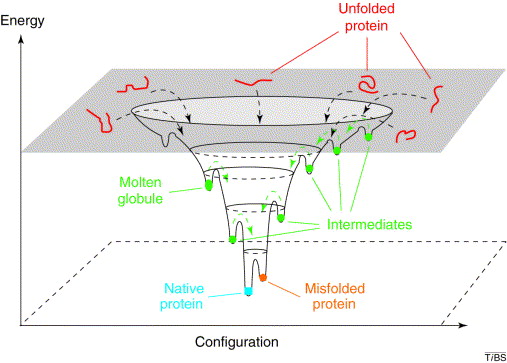
One can imagine that in vivo native structures of proteins look-alike the lowest free energy conformation, i.e., the global energy minimum. That is the basis of the funnel model of protein folding, which assumes that the number of possible conformations is reduced when a local energy minimum is achieved, constituting a path for the folding process.
In conclusion, prediction of protein structures is possible, as protein folding relies only on the protein sequence, but it will require virtually infinite time and computational resources.
Homology modeling is one of the easiest tricks to bypass that limitation. Basically, the strategy is adding all the possible extra information to the amino acid properties, namely the evolutionary conservation of sequences and structures.
Very often, before generating models you already have some information about your protein. For instance, if it is an enzyme you may have spotted the catalytic residues or substrate-interaction region. It is also advisable to check the literature, particularly if there is a companion paper of the related PDB structure(s) that may be available or that you can eventually find and use as the template(s) for modeling.
2 Homology modeling: Conservation is the key
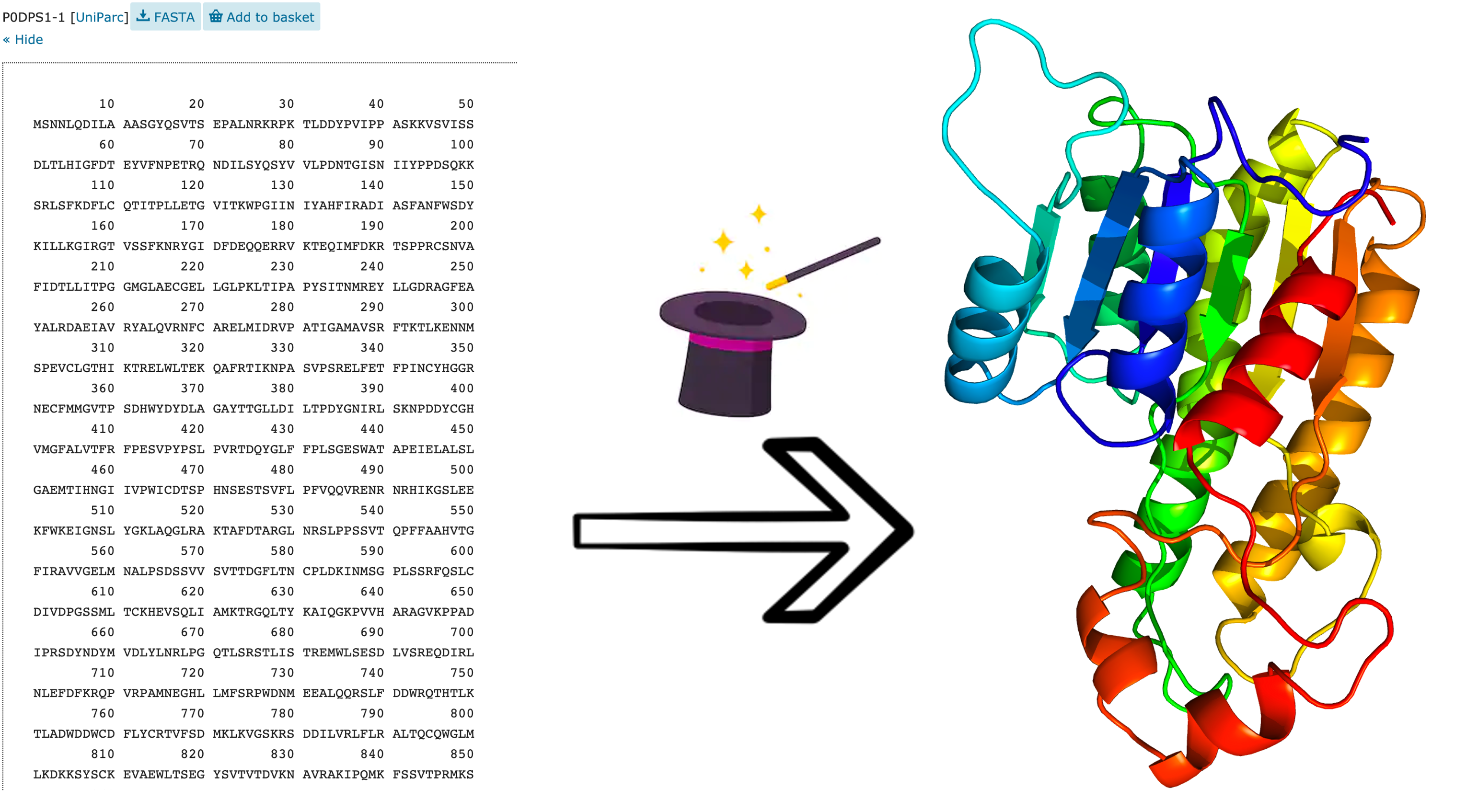
So, we want to go from the sequence to the structure. But, how do we do it?
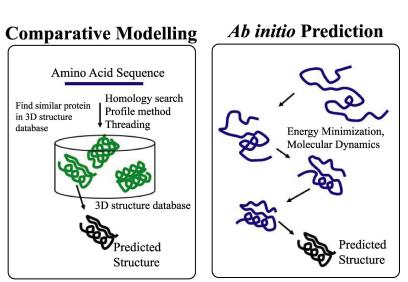
Traditionally and conceptually, the prediction of protein structures can be addressed from two different perspectives: comparative modeling and ab initio prediction. In the comparative modeling approach, one can predict 3D structure for protein sequences only if their homologs are found in the database of proteins with known structures. Obviously, the identification of such homologs is the keyword here. Until recently, most of the evolution in protein modeling was driven by the evolution of methods to identify distant sequence similarities that would reflex similar protein folds.
The accuracy of homology modeling will be limited by the availability of similar structures, ab initio predictions are limited by the mathematical models and computational resources, being often useful only with small peptides.
To maintain structure and function, certain amino acids in the protein sequence suffer a stronger selective pressure, evolving either slower than expected or within specific constraints, such as chemical similarity (i.e., conservative substitutions). Thus, homology modeling approaches assume that a similar protein sequence involves a similar 3D structure and function. At the same time, although the number of published sequences and structures continually increased, the number of unique folds remains almost steady since 2008.
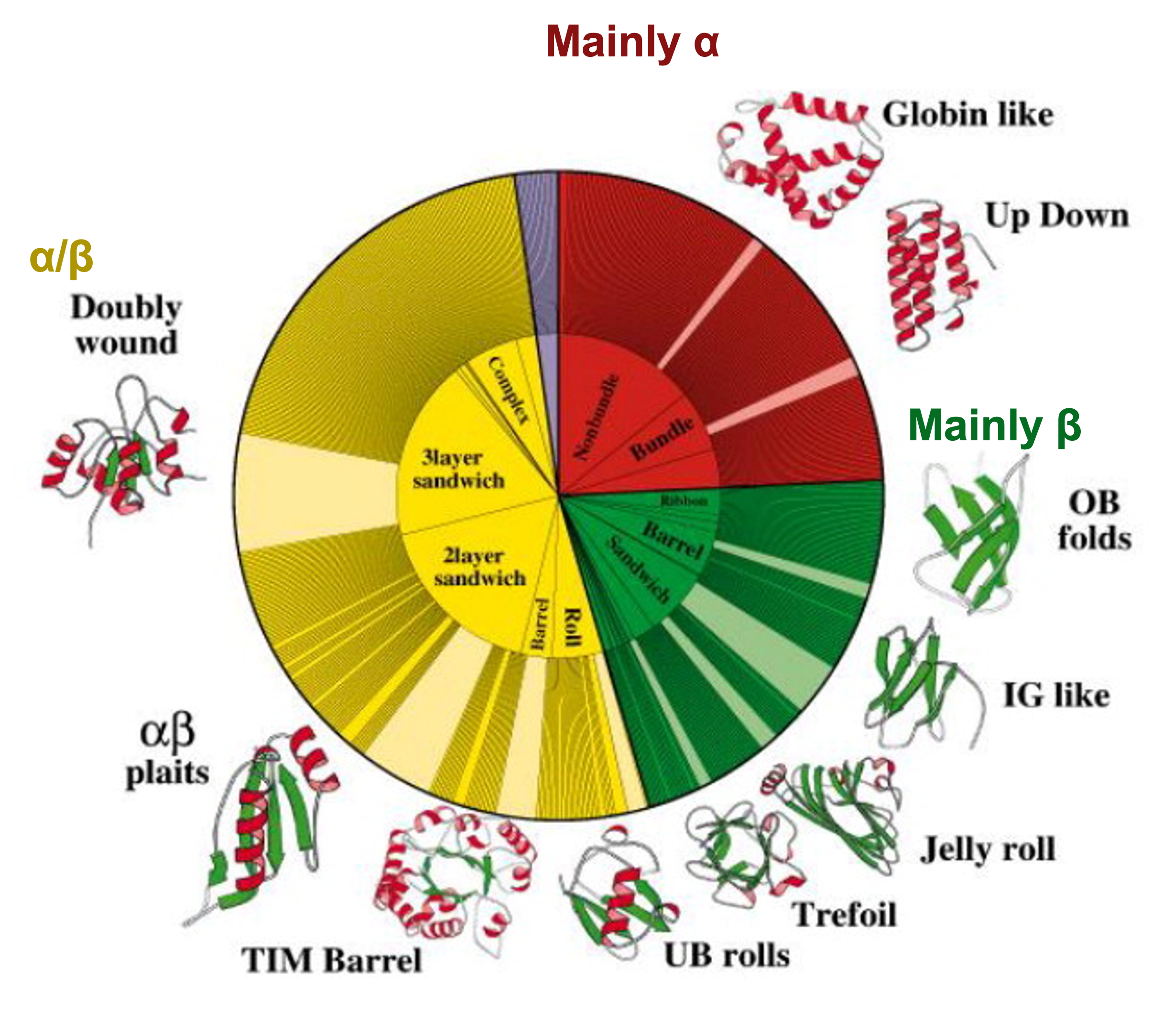
That implies that the space of protein sequences is much larger than the space of structures. This has been exploited by some structural databases, like Scop2 or CATH, that use hierarchical classifications of structures into very few categories of different structures.
In conclusion, protein structures are more conserved than sequences, which allows for model construction by comparison of related proteins. Biological sequences evolve through mutation and selection and selective pressure is different for each residue position in a protein according to its structural and functional relevance. Sequence alignments attempt to tell us the evolutionary story of proteins.
3 Homology modeling in four steps
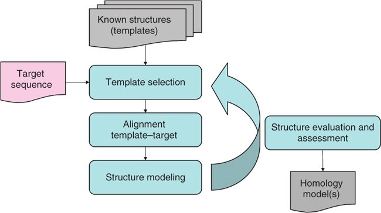
A basic workflow of homology modeling requires three steps: (1) identification of the best-suitable template, (2) alignment of the query sequence and the template, and (3) construction of the model. These steps can be addressed with diverse methodological alternatives and can provide different outputs that must be assessed (step 4) in order to decide the best solution for each step. For a more detailed review on homology modeling, I suggest reading Haddad, Adam, and Heger (2020).
In this course, we are focusing on end-to-end modeling with SWISS-MODEL (Waterhouse et al. 2018), a fully automated modeling server that allows you the construction of models without the requirement of a strong bioinformatics background or coding skills. Rather than provide just a recipe to make a model in two clics, the goal is to understand the process behind, while doing exercises with selected examples
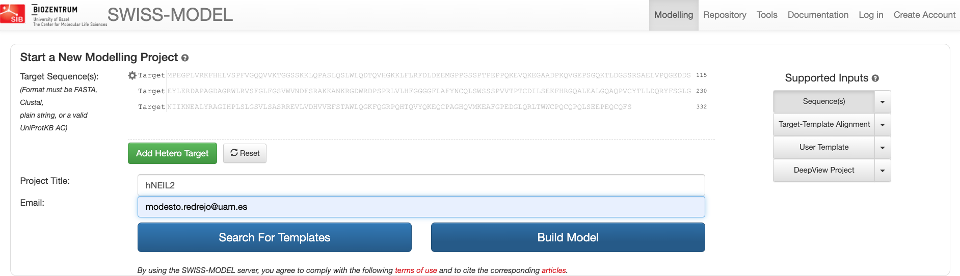
SWISS-MODEL has different supported inputs. Usually, you provide a single sequence query for template searching, but you can bypass this step and provide directly the template you want to use and even a template and a custom alignment. We will start with the first alternative.
As an example, we are modeling a human DNA repair protein, NEIL2, and a viral DNA polymerase (HAdV-2 pol).
3.1 Step 1+2. Template search and align.
3.1.0.1 Where can we search?
Template searching consists in finding a protein with known structure that has a sequence similar to our protein. As we mentioned above, the RCSB Protein Data Bank (PDB) is the largest database of protein structures, so we can search templates by comparing the sequence of our protein with the sequence of all the proteins in PDB. However, PDB was constructed to contain all the macromolecular structures, not for searching templates for modeling. Similar to other end-to-end software, SWISS-MODEL has its own curated database, called SMLT (SWISS-MODEL template library). This is based on PDB, updated weekly, and also annotated and indexed to boost searches. As of 6 July 2022, SMLT contains 133,049 unique protein sequences that map to 332,864 biological units (protein structures classified by their quaternary structure).
Now, you first need your sequences. A protein sequence database like Uniprot or NCBI Protein, is a good place for this task.
If you want to cheat yourself, I give you the HAdV-2pol and NEIL2 sequences.
3.1.0.2 How can we search accurately and fast?
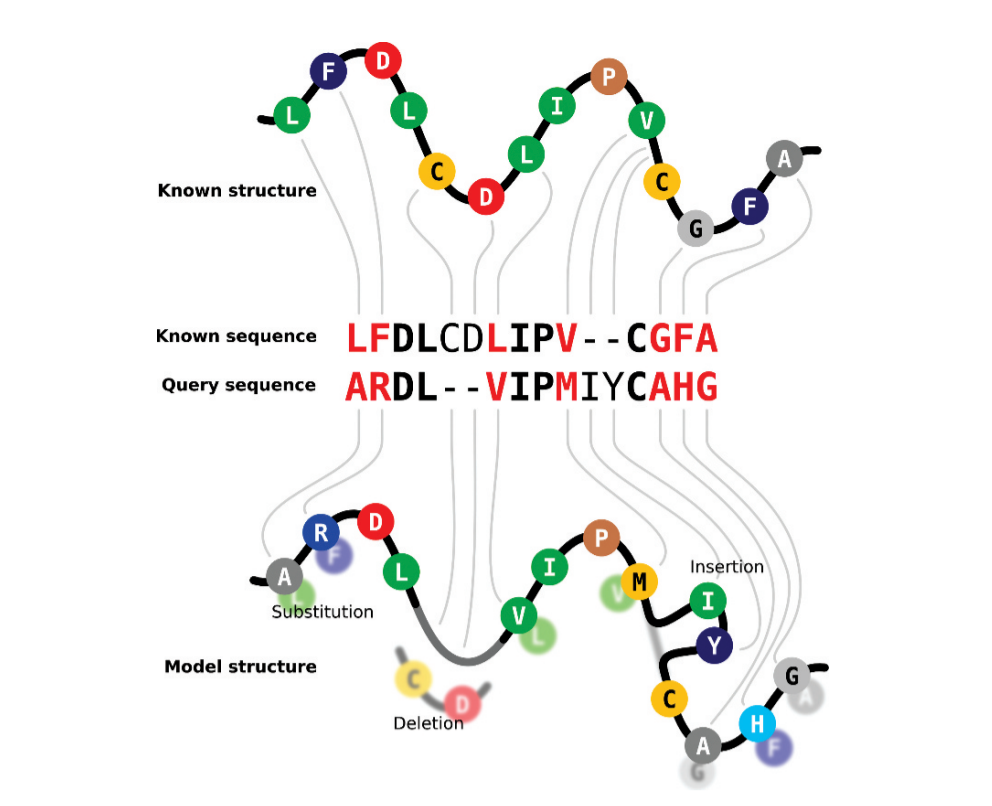
Finding templates require comparing sequences, thus an accurate and powerful alignment method is essential. Comparing one protein sequence with a whole database is time-consuming, as you will compare with totally unrelated proteins, which is a loss of resources. Two basic improvements increased the template search capacity, (1) the introduction of secondary structure (SS) by comparing SS predictions of the query protein and the secondary structures of the protein database, and (2) the use of profiles to make easier the comparison. Profiles are a mathematical way to summarize a multiple sequence alignment in which the frequency of each amino acid at each position is quantified. A particular type of profiles, the Hidden Markov Models (HMM) are very well suited to search databases for similar sequences. HMMs include amino acid insertions and deletions, meaning that they can model entire alignments, including divergent regions. This allows the identification of highly conserved positions that not only define the protein function but also the fold. For instance, glycine residues at the end of each beta-strand or a pattern of polar residues that favors alpha-helices. A previous comparison of the query sequence with a database of sequences will allow us to include evolutionary information about it. Therefore, we moved from a requirement of >30% identity to obtain good models before the implementation of profiles, to good models even with ⁓20% identity or below. Moreover, the generation of profiles also facilitates clustering of the search database, reducing search time. The implementation of these capacities led to the implementation of the so-called fold recognition in homology modeling.
Template searches in SWISS-MODEL are carried out with HHblits (Remmert et al. 2011), a specific profile-profile method. We could search for templates also with Blast or other profile-profile methods, like Psi-BLAST, HHPred, or JackHMMER. Then templates are ranked by two different numeric parameters ranged between 0 and 1: GMQE (global model quality estimate) and QSQE (quaternary structure quality estimate). Briefly, GMQE uses probability functions to assess several properties of the target-template alignment (sequence identity, sequence similarity, HHblits score, the agreement between predicted secondary structure of target and template, the agreement between predicted solvent accessibility between target and template; all normalized by alignment length) to predict the expected quality of the resulting model. QSQE assesses the oligomeric state probability of the model.
Now, paste your sequences and click on Search.
Note: If you click on “Build Model”, it will directly use the top-ranked template, so you’ll miss some fun, but you can go back for that later on if you change your mind.
Before building the models, could you foresee which of our queries will give rise to a better model? Why?
3.2 Step 3. Model Building.
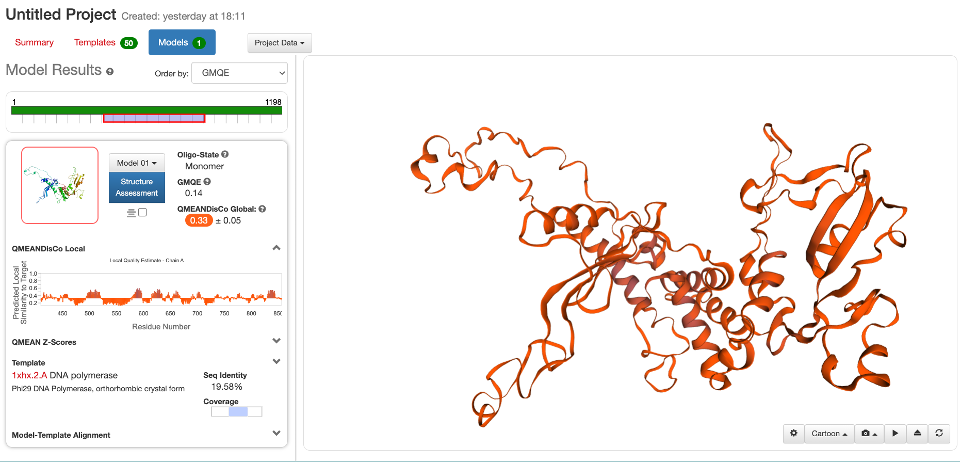
By default, SWISS-MODEL will provide 50 ranked possible templates. The output also contains information about the method and resolution of the templates, the % of identity (and alignment coverage) with the query sequence, and the GMQE and QSQE.
The top template is marked by default and it likely will give the best model, but it is also interesting to try some alternative templates depending on the downstream application of the model (see below). For instance with a different substrate/cofactor that can have a key role in the protein function or with different coverage or % identity.
Once the template(s) is selected, model coordinates are constructed based on the alignment of the query and template sequence. SWISS-MODEL uses a fragment assembly, as well as RosettaCM, another very well-known homology modeling algorithm by the Baker lab, implemented in Rosetta software and the Robetta server. Other programs, like Modeller, are based in the satisfaction of spatial restraints (Janson et al. 2019).
Fragment assembly will use the template core backbone atoms to build a core structure of the model, leaving non-conserved regions (mostly loops) for later. Then, it will find compatible fragments in a dedicated loop database and also use ab initio building or missing loops.
Then, side chain of non conserved amino acids in undertook. The goal is findig the most likely side chain conformation, using template structure information, rotamer libraries (from known protein structures) and energetic and packaging criteria. If many side chains have to be placed in the structure it will lead to a “chicken and egg problem”. Thus, the more residues correctly positioned, the best model. That means that identification of hydrogen bonds between residues side chains and between side chains and the backbone reduce the optimization calculations.
Finally, a short energy minimization is carry out to reduce the unfavorable contacts and bonds by adapting the angle geometries and relax close contacts. This energy minimization step or refinement can be useful to achieve better models but only when the folding is already accurate.
3.3 Step 4. Result assessing.
Output models are colored in a temperature color scale, from navy blue (good quality) to red (bad quality). That can help us to understand our model in a first sight. Also, this is an interactive site and you can zoom-in, zoom-out the model. Many other features are available to work on your model. For instance, you can compare multiple models, you can change the display options. You can also download all the files and reports on the “Project Data” button.
There is also a “Structure Assessment” option. This provide you a detailed report of the structural problems of your model. You can see Ramachandran plots that highlight in red the amino acid residues with abnormal phi/psi angles in the model and a detailed list of other problems.
The GMQE is updated to the QMEAN Zscore and QMEANDisCo (Studer et al. 2020). The QMEAN Z-score or the normalized QMEAN score indicates how the model is comparable to experimental structures of similar size. A QMEAN Z-score around 0 indicates good agreement, while score below -4.0 are given to models of low quality. Besides the number, a plot shows the QMEAN score of our model (red star) within all QMEAN scores of experimentally determined structures compared to their size. Overall, the Z-score is equivalent to the standard deviation of the mean.
The QMEANDisCO is a single parameter that combines statistical potentials and agreement terms with a distance constraints (DisCo) to provide a consensus score. DisCo evaluates consistencies of pairwise CA-CA distances from a model with constraints extracted from homologous structures. All scores are combined using a neural network trained to predict per-residue scores.
QMEANDisCo can be used to analyze models obtained with other methods in order to make them comparable. There are other model assessing tools commonly used to assess protein models, like MoldFold (McGuffin et al. 2021) or VoroMQA (Olechnovič and Venclovas 2017).
Another key parameter that you should know if you want to compare protein strutures is the alpha carbon RMSD (root mean square deviation of the alpha carbons), sometimes referred in the protein field only as as RMSD. Any protein structural alignment will give you this parameter as an estimation of the difference of the structures. You can align structure with many online servers, like FATCAT2 or using molecular visualization apps, like ChimeraX or Pymol (also available here).
Which model is better? Which regions are more difficult to model? Why?
3.4 Corollary: What can I do with my model and what I cannot?
A big power entails a big responsibility. The use of models entails a precaution and a need for experimental validation. However, knowing the limitations of our model is required for a realist use of it; and limits are defined by the model quality.
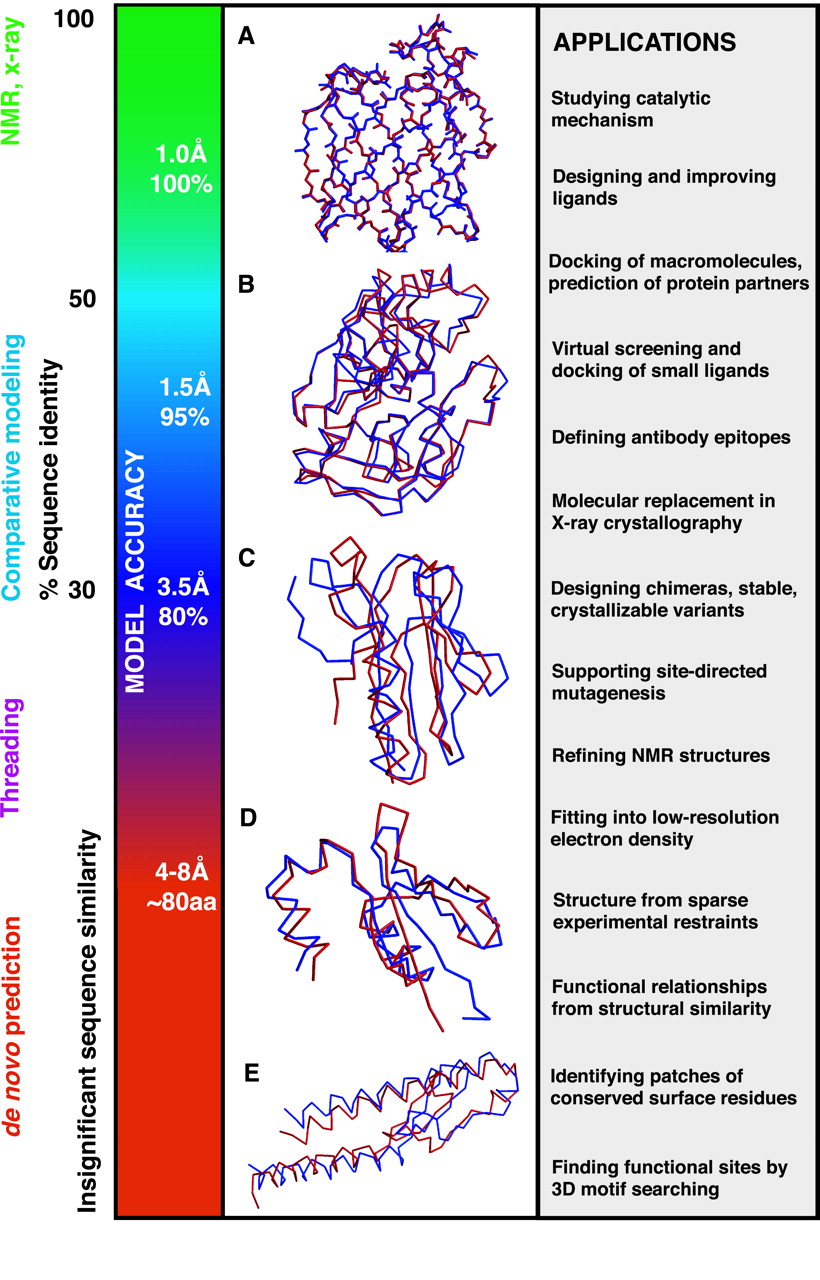
The accuracy of a comparative model is related to the percentage sequence identity on which it is based. High-accuracy comparative models can have about 1-2 Å root mean square (RMS) error for the main-chain atoms, which is comparable to the accuracy of a nuclear magnetic resonance (NMR) structure or an x-ray structure. These models can be used for functional studies and the prediction of protein partners, including drugs or other proteins working in the same process. Also, for some detailed studies, it would be convenient to refine your model by Molecular Dynamics and related methods towards a native-like structure. I suggest checking the review by Adiyaman and McGuffin (2019) on this topic.
On the contrary, low-accuracy comparative models are based on less than 20-30% sequence identity, hindering the modeling capacity and accuracy. Some of these models can be used for protein engineering purposes or to predict the function of orphan sequences based on the protein fold (using Dali or Foldseek).
As mentioned above, it also advisable to check the template structures and read the papers describing them in order to squeeze all the information from your model.
What do you think you could use our models of NEIL2 and HAdV-2pol?