Introduction
Goals and Warnings
Structural Bioinformatics is a broad discipline that covers structural and computational biology, from visualization and analysis of the structure of biomacromolecules to protein modeling and molecular docking. Powered by great technical advances, the field has experienced a great revolution in the last decade. The increase of experimental capacities to analyze the structure of proteins and other biological molecules and structures (see Callaway (2020)) and the development of Artificial Intelligence (AI)-assisted structure prediction boosted the capacity of life-science researchers to address a wide variety of questions regarding proteins diversity, evolution and function. The implications of this revolution in biology, biotechnology, and biomedicine are still unforeseen.
For a short introductory course on protein modeling, I propose the following three basic objectives:
Identify the main applications and limitations of the prediction of protein structures in biomedicine and biotechnology.
Become familiar with classic and state-of-the-art protein modeling methods.
Basic understanding of the resulting output of a protein modeling experiment and how to evaluate and eventually improve the model quality.
Warning for future structural biologists
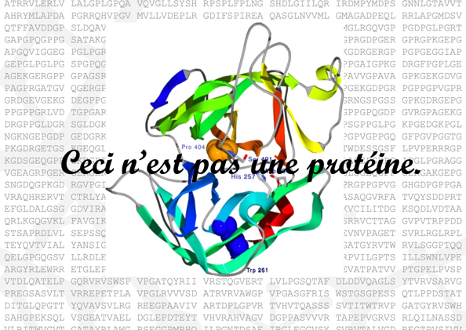
The surrealist Belgian painter René Magritte created a collection of surrealistic paintings entitled La trahison des images (1928–1929). The most renowned of those paintings show a smoking pipe and the following caption underneath: “Ceci n’est pas une pipe” (This is not a pipe). Yes, indeed! It is actually the painting of a pipe.
Similarly, a picture of a protein, or a PDB file with the coordinates of a protein structure, is not a protein. It is a representation of ONE structure. Even experimentally determined structures have two main limitations that we should always keep in mind: (1) they are a fixed structure whereas proteins in vivo are flexible and dynamic and (2) they are subjected to experimental error and they often contain regions of low reliability. Moreover, even experimentally obtained macromolecular structures are to some degree models, with a variable ratio between experimental data and computational prediction to match the experimental data (X-ray diffraction, cryo-EM density maps, NMR, SAXS, FRET…) with previously known structures or prediction models. That does not mean that protein structures are useless, they can be very useful, but we must be aware of the limitations as well as the applications.
Before going forward: Protein Structure 101
Although you can make some protein modeling without being an expert in structural biology, a basic understanding of protein structure is strongly advisable. Over the years, I noticed that graduate students in biology, biomedicine, and related fields have a very different background on protein structure. If you want to review and update your background on protein structure, I recommend you the great review by Stollar and Smith (2020) and the Wikipedia articles on protein structures (https://en.wikipedia.org/wiki/Protein_structure), which constituted my main source for this brief section.
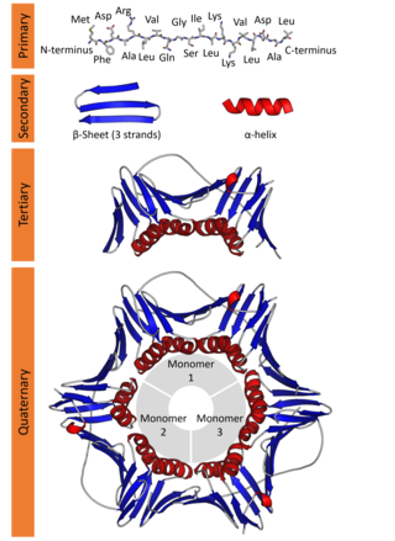
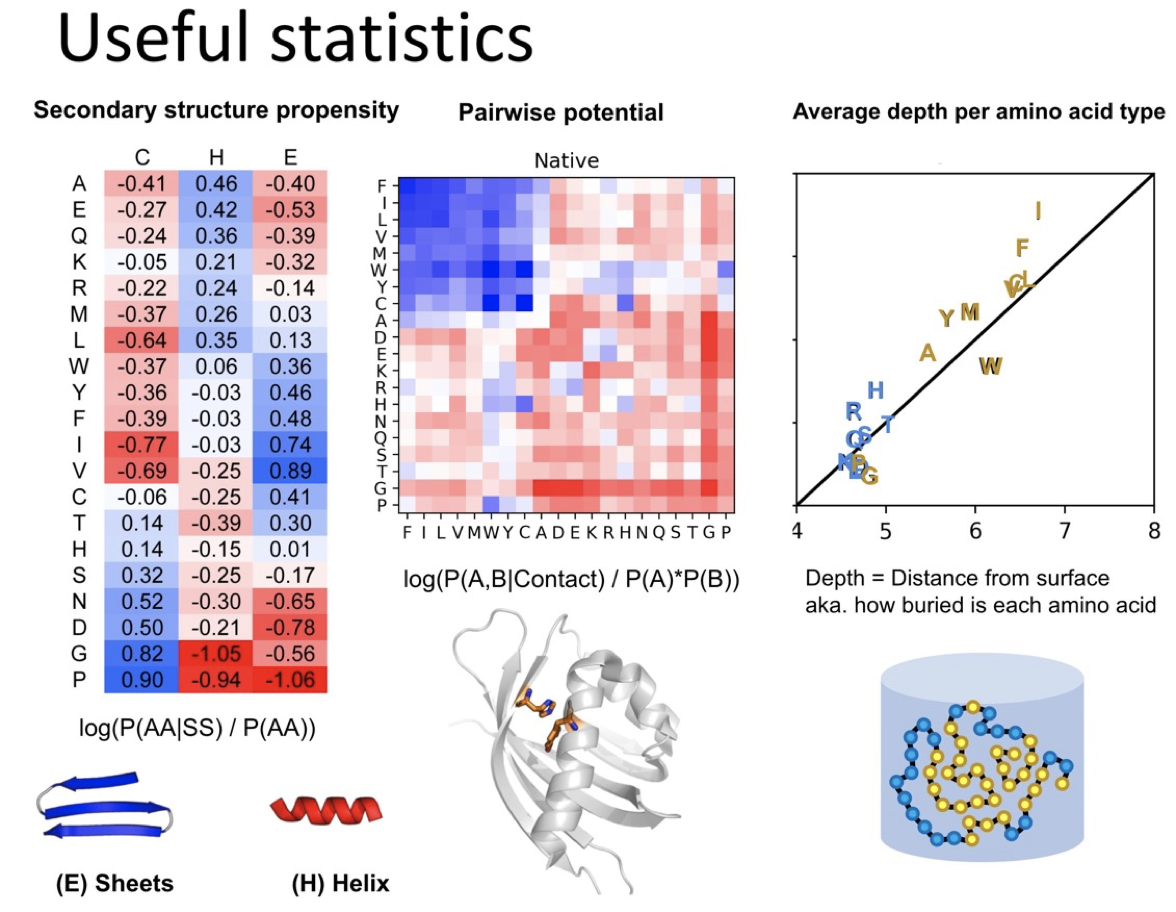
Proteins are key components of life, playing key roles in almost any possible vital function, either as structural, or scaffolding elements or as active enzymes that catalyze metabolic reactions. Proteins are built as polymers of amino acids and the sequence of amino acids of a particular protein can be also called the primary structure of the protein. Amino acid chains can spontaneously fold up into three-dimensional structures, mostly stabilized by hydrogen bonds between amino acids. The amino acid sequence determines different layers of 3D structure. Each of the 20 natural amino acids has different physicochemical properties that affect its preferred conformation. Thus, the first level of folding is called secondary structure, forming common patterns as we will see in a moment.
These stretches of secondary structure patterns can fold in 3D due to interactions between the side chains of amino acids. This is called protein tertiary structure. Finally, two or more individual peptide chains can form multisubunit proteins that have the so-called quaternary structure.
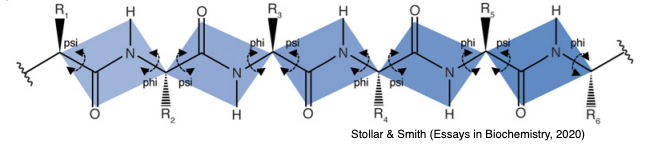
It should be noted that the peptide bond itself cannot rotate as it has a double bond-like character. Therefore, rotation can only occur about the bond between the Cα and the C = O group, (the phi (φ) angle) and the Cα and the NH group, (the psi (ψ) angle). In fact, the polypeptide backbone chain is composed of a repeating series of two rotatable bonds followed by one non-rotatable (peptide) bond. However, not all 360º of the psi and phi angles are possible as neighboring sidechains can clash due to steric hindrance. For certain angles and amino acid combinations, the atoms cannot be in the same physical place and this partly explains why some amino acids have a higher propensity (likelihood) to form different types of secondary structures.
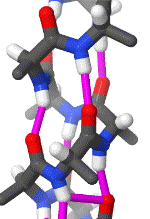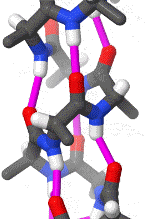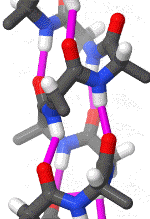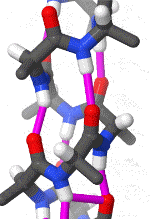
Within these restraints, the two principal local conformations that avoid steric hindrance and maximize backbone–backbone hydrogen bonding are the α-helix and the β-sheet secondary structures. The α-helix is a right-handed coil in which backbone NH group hydrogen bonds to the backbone C = O group of the amino acid located four residues earlier along the protein sequence. This results in a polypeptide chain that twists in a regular coil shape with the R-groups pointing outwards away from the peptide backbone. It takes approximately 3.6 residues to complete a full turn of a helix.
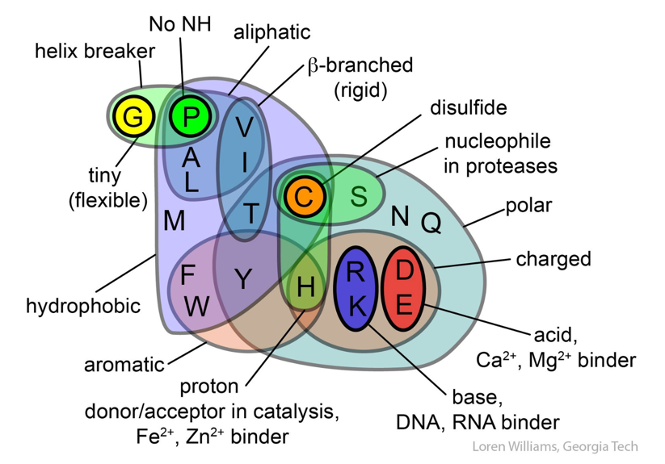
Different amino-acid sequences have different propensities for forming α-helical structures. Methionine, alanine, leucine, glutamate, and lysine have especially high helix-forming propensities, whereas proline and glycine have poor helix-forming propensities. Proline either breaks or kinks a helix, both because it cannot donate an amide hydrogen bond (having no amide hydrogen), and also because its bulky sidechain interferes sterically with the backbone of the preceding turn. However, proline is often seen as the first residue of a helix, it is presumed due to its structural rigidity. At the other extreme, glycine also tends to disrupt helices because its high conformational flexibility makes it entropically expensive to adopt the relatively constrained α-helical structure.
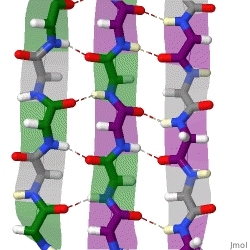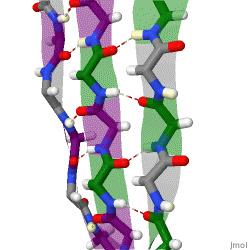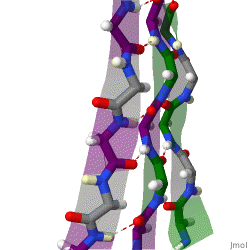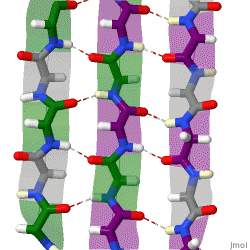
β-sheets are composed of two or more extended polypeptide chains called β-strands that run alongside each other. They can be arranged in either a parallel or antiparallel manner. The residues arrange themselves in a regular zigzag manner with the adjacent peptide bonds pointing in opposite directions. In this arrangement, the NH group and the C = O group of each amino acid are hydrogen-bonded to the C = O group and NH group respectively on the adjacent strands. Chains can run in opposite directions, forming an antiparallel β-sheet, or in the same direction, forming a parallel β-sheet. Sidechains from each of the residues point away from the sheets and alternate in opposite directions between residues. It is common to see a pattern of alternating hydrophilic and hydrophobic residues in the primary structure, giving the β-sheets hydrophilic and hydrophobic faces.
Large aromatic residues (tyrosine, phenylalanine, tryptophan) and β-branched amino acids (threonine, valine, isoleucine) are favored to be found in β-strands. As in the case of α-helixes, β-strands are often ended by glycines, which are especially common in β-turns (the most common connector between strands), as amino acids with positive φ angles.
Playing with secondary structures
There are a few online alternatives to model any peptide sequence and quickly see the effect of amino acid composition in the secondary structure. One of the best-known is Foldit (www.fold.it, Miller et al. (2020)), a gaming platform for biochemistry and structural biology teaching. It is a highly recommended alternative for most courses related to protein structure.
In this course we are going to try a more recent proposal, recently twitted by Sergey Ovchinnikov (see https://twitter.com/sokrypton/status/1535857255647690753). It is based on ColabFold (see https://github.com/sokrypton/ColabFold and Mirdita et al. (2022)), an Alphafold2 (see Jumper et al. (2021)) free notebook in Google Colab notebook. All you need is a Google account and the following cheatsheet.
Now go to ColabFold Single: https://colab.research.google.com/github/sokrypton/af_backprop/blob/beta/examples/AlphaFold_single.ipynb
Construct some small proteins and compare the output. Note that the first model will take 3-5 min, but the others will be faster. I provide here some interesting examples (IUPAC one-letter amino acid code):
AAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAA
KKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKK
PVAVEARENGRLAVRVEGAIAVLIRENGRLVVRVEGG
PELEKHREELGEFLKKETGIAVEIRENGRLEVRVEGYTDVKIEGGTERLKRFLEEL
ACTWEGNKLTCA
Answer the following questions:
- Why is a poly-K more stable (dark blue) than a poly-A?
- Could you predict the structure of a poly-V or a poly-G?
- What would happen if you introduce a K5W in the structure number 2? and in the 4?
Now, try to create peptides with:
- Two helices
- A four-strands beta-sheet.
- Alpha-beta-beta-alpha.